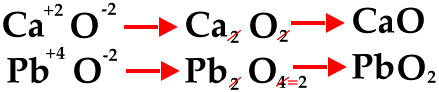How do you write the formula for zinc acetate?
1 Answer
Zinc acetate is an ionic compound. The easiest way to learn to write such a formula is to learn to write the formula of the cation first, and then the formula for the anion, then crisscross their charges.
Explanation:
The bond that forms zinc acetate is ionic. Zinc forms 2+ cations, with the formula
Since the compound needs to be neutral, the total positive charge must equal the total negative charge, so the formula for zinc acetate requires one
The formula is then
A very easy method for writing ionic formulas is called the crisscross method. It is shown in the diagram below.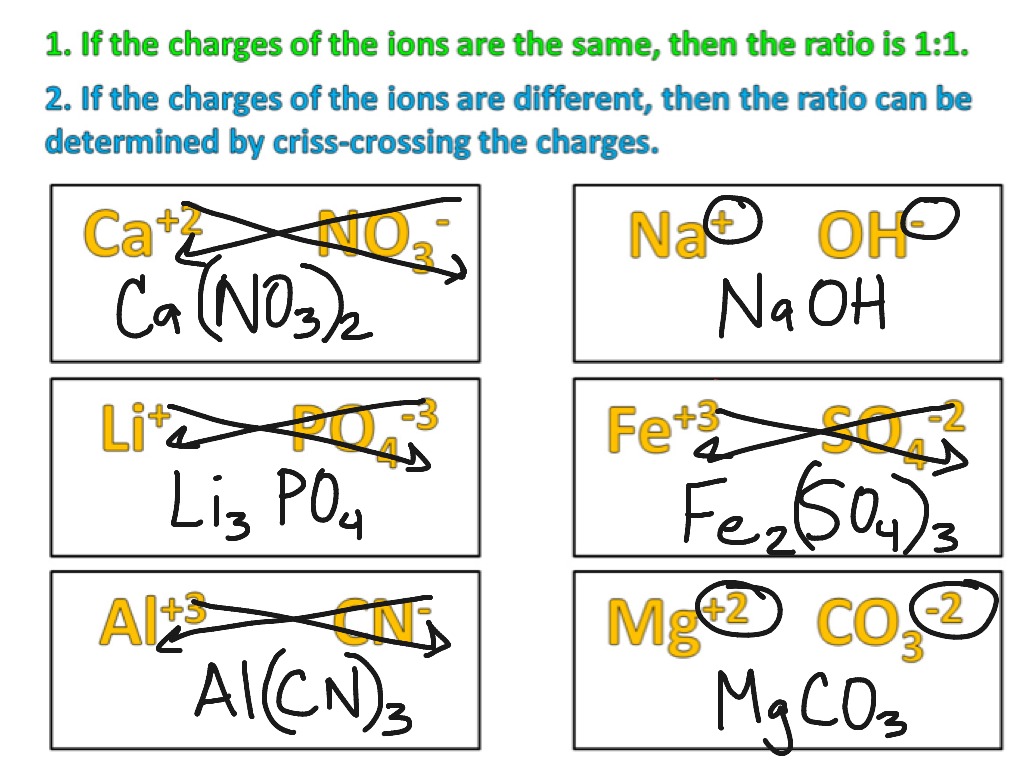
Since a chemical formula for an ionic compound must indicate the lowest whole-number ratio of ions, sometimes after crisscrossing you must reduce the ratio.