Question #25fad
1 Answer
Cool things happen, depending on the solid!
In general higher pressures will force the solid to adopt more and more compact crystal structures. You find this information from a phase diagram, relating pressure and temperature. Let's look at the phase diagram for water:
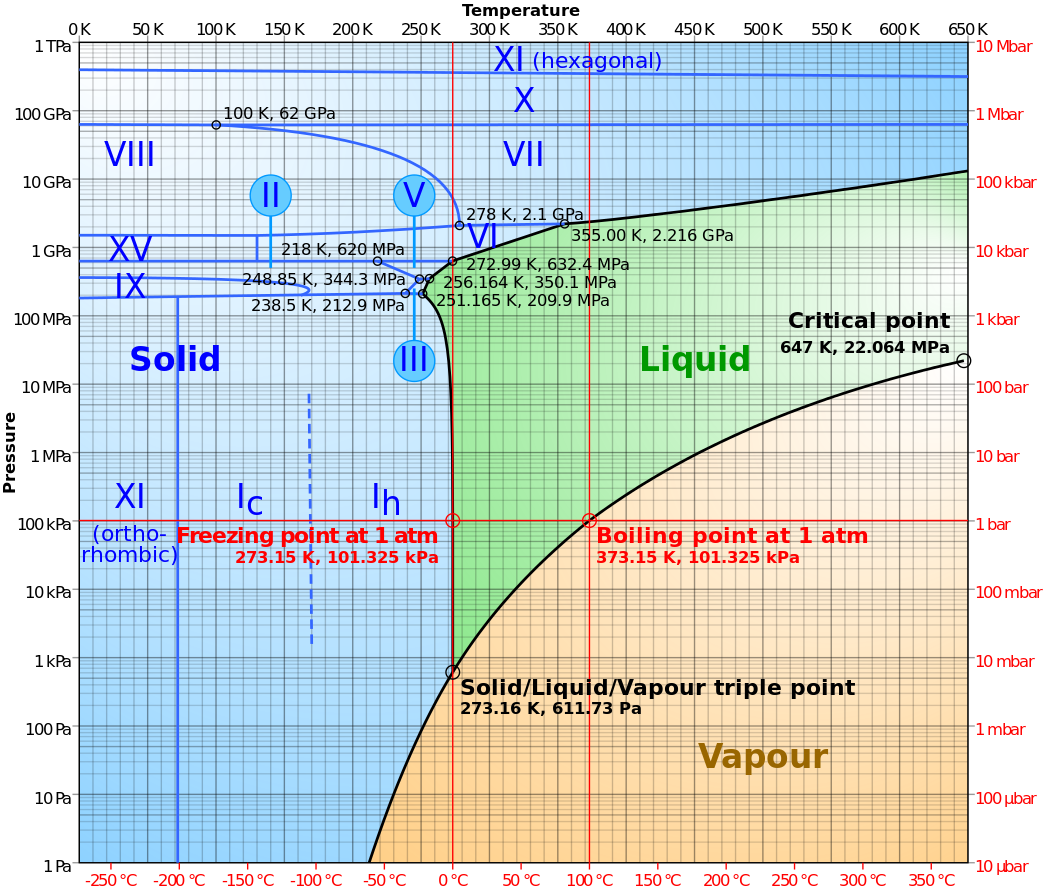
Let's take the line at -50 C. Follow it up and you are seeing increasing pressure. At about two thousand atmospheres (212 MPa), Ice 1h converts to ice II. Then Ice V at ~400 MPa, then Ice XV at ~ 620 MPa. Ice VIII occurs at about 2 GPa (20,000 atm), followed by Ice X at 62 GPa. Finally, above 400 GPa you get Ice XI. Each of these ice species has a different crystal structure. Some are actually more compact than liquid water at room temperature!
If we keep increasing pressure beyond that, ice will turn into a metal (at about 1.5 TPa.
Beyond that, at some point you are going to crush the atoms together forcing fusion of elements lighter than iron, forming iron. If you keep on applying pressure beyond that you will eventually collapse the electrons into the protons forming pure neutronium. This is what happens at the heart of a supernova!

