Question #1efaf
3 Answers
A neutral fluorine atom has seven valence electrons. You can determine the number of valence electrons for any element of the main group of elements by using their group numbers. The main group consists of all of the elements EXCEPT the transition and inner transition elements. The number of valence electrons for the main group elements can be determined by their group numbers as follows:
Group 1/IA: 1 valence electron
Group 2/IIA: 2 valence electrons
Group 13/IIIA: 3 valence electrons
Group 14/IVA: 4 valence electrons
Group 15/VA: 5 valence electrons
Group 16/VIA: 6 valence electrons
Group 17/VIIA: 7 valence electrons (of which fluorine is a member)
Group 18/VIIIA: 8 valence electrons (except helium, which has 2 valence electrons)
Fluorine has 7 Valence electrons.
-
Valence electrons are those electrons which are present in the outermost shell of an atom.
-
Fluorine has atomic number 9. Atomic number is the number of protons present in the nucleus of an atom.
-
In every stable (Neutral) atom the number of electrons are equal to the number of protons.
-
Therefore, the number of electrons in an atom of fluorine are 9.
-
The electronic configuration of fluorine is:
E.C:- k - 2 , L-7
The last shell of fluorine has 7 electrons. Therefore there are 7 valence electrons in an atom of fluorine.
Fluorine has
Explanation:
Fluorine is located in group 17, period 2 of the periodic table, and has an atomic number equal to 9.
This means that a neutral fluorine atom has a total of 9 electrons surrounding its nucleus.
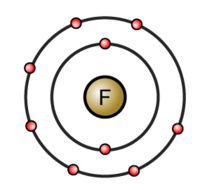
Bu how many of those 9 electrons are valence electrons?
You know that valence electrons are located in the outermost shell of an atom. You can use fluorine's electron configuration to help you determine how many electrons are located in the outermost shell
#"F": 1s^2 2s^2 2p^5#
The first energy level has 2 electrons located in the 1s-orbital and the second energy level, which in fluorine's case is the outermost energy level, has 7 electrons, 2 in the 2s-orbital and 5 in the 2p-orbitals.
This means that fluorine has a total of 7 valence electrons.




