Question #d3e85
1 Answer
The bonds above and below any two adjacent carbon atoms are behind the plane of the paper.
The carbon chain has a eclipsed conformation.
The two chiral atoms are in the plane of the paper. The horizontal bonds are coming out of the paper.
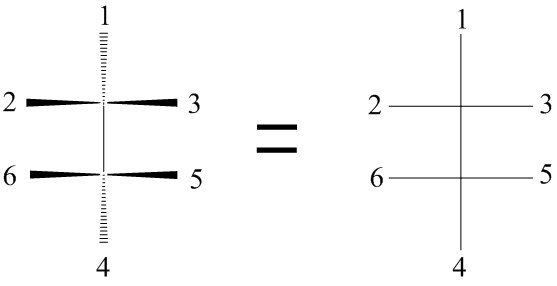
The carbon atoms have a tetrahedral geometry, so the vertical bonds to groups 1 and 4 must be behind the plane of the paper.
When you have a longer vertical chain, you look at any two adjacent atoms.
Those two atoms are in the plane of the paper, and the two atoms directly above and below them are behind the plane of the paper.
You keep the same orientation as you move up and down the chain.

The chain is arch-shaped, as if it you wrapped around around a cylindrical tube.
When you flatten the structure onto the surface of the cylinder, you get the Fischer projection of the molecule.

