What is the chemical formula for calcium oxide?
1 Answer
The formula for calcium oxide is CaO.
Explanation:
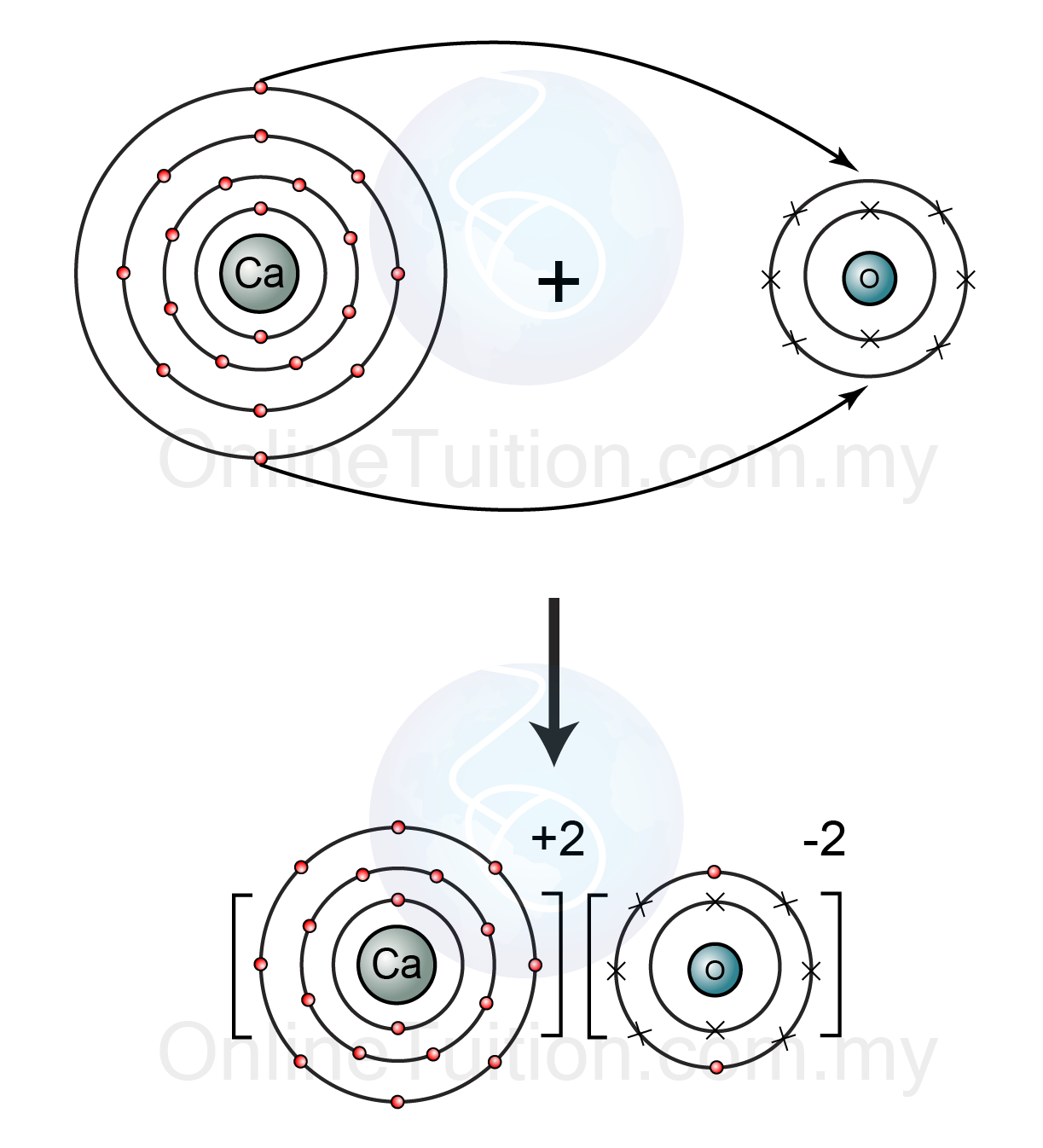
Calcium oxide is an ionic compound composed of the metal calcium and nonmetal oxygen.
Calcium is an alkali earth metal (group 2/IIA). The atoms of the alkali earth metals have two valence electrons, which they lose when forming ions. Therefore, calcium cations have a charge of
Oxygen is in group 16/VIA and the atoms of all elements in this group have six valence electrons, and need two more to have an octet and become stable. Therefore, oxide anions have a charge of
The overall charge of an ionic compound is zero. Since the charges of the calcium and oxide ions are equal but opposite, the calcium and oxide ions form a
 http://spmchemistry.onlinetuition.com.my/2013/10/ionic-bonding.html
http://spmchemistry.onlinetuition.com.my/2013/10/ionic-bonding.html

