What is the difference between reversible and irreversible work?
1 Answer
A reversible process is one that can be undone precisely to exactly give back the previous state. It is the most efficient pathway that the process can go through.
For example, let's say we compared doing work in two stages compared to doing it in very small increments.
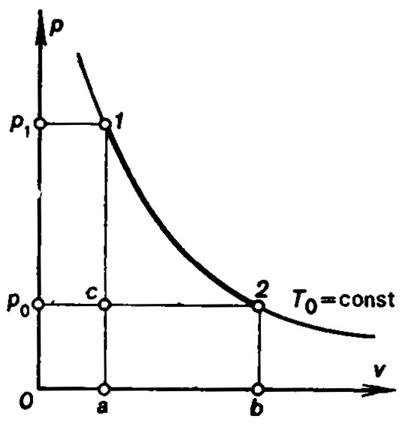
If you allow a gas to do work in two stages, it can expand under constant pressure, and then you allow the pressure to decrease naturally. Then, you can allow it to expand again at constant pressure, and then you allow the pressure to naturally decrease again.
Alternatively, you could allow the gas to expand and do work while slowly decreasing the pressure. That allows the gas to slowly expand in small increments, rather than expand quickly in two bursts.
The work done is
You can see that you move along a shorter distance along the curve with the second way. This can be notated as
This second way is called reversible work. The first way is not reversible because now the gas behaves differently at this lower pressure. When you alter the pressure slowly, the gas has time to readjust as it needs to, allowing it to do the reverse much more easily.

