When solid A is added to water, the solution conducts electricity. When solid B is added to water, it does not conduct electricity. Why is this?
1 Answer
Dec 1, 2014
Solid A is a water soluble ionic compound which dissolves in water by dissociating into its individual positively charged ions and negatively charged ions, forming an electrolytic solution that can conduct electricity,
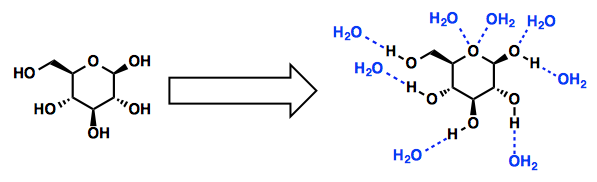
Solid B is either an insoluble ionic compound, a nonpolar substance, neither of which will dissolve in water, or it is a polar molecular (covalent) compound that dissolves in water without dissociating.and forming individual ions. Water is a polar molecule and will dissolve polar solutes. For example, the sugar glucose is a polar molecule and dissolves in water. Glucose molecules have many hydroxyl (OH) groups that attract the water molecules. The water to water hydrogen bonds break and glucose-water hydrogen bonds form.