Question #c22fc
1 Answer
You can determine which compounds are the solutes and which are the precipitates by using solubility rules (meaning solubility in water) or a solubility table. The solutes will be soluble, and therefore will be in aqueous solution, as indicated with the symbol (aq) written after the chemical formula of the solute. The precipitate will be insoluble, which is indicated with the symbol (s) written after the chemical formula of the precipitate. The following diagram is an example of a solubility table.
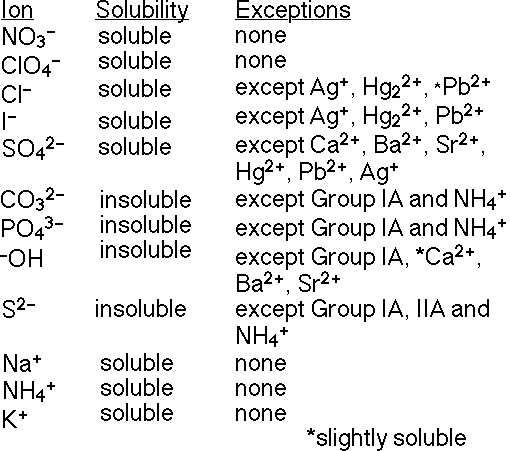
Example. Which compounds in the following reaction are soluble or insoluble in water?
Check the solubility table to determine which compounds are soluble or insoluble in water.
So now we can write the equation with the symbols that indicate the state of each compound.

