Question #a738e
2 Answers
The difference between polar and non-polar lies in the final charge determined by the covalent bond. Polar molecules will have a net dipole moment which results from partial positive and partial negative charges that arise from a difference in electronegativity between the molecule's atoms.
Electronegativity describes an atom's ability to attract the electrons in a bond towards itself. Non-polar molecules come about either when two elements have similar or equal electronegativities, or when the dipoles balance each other out.
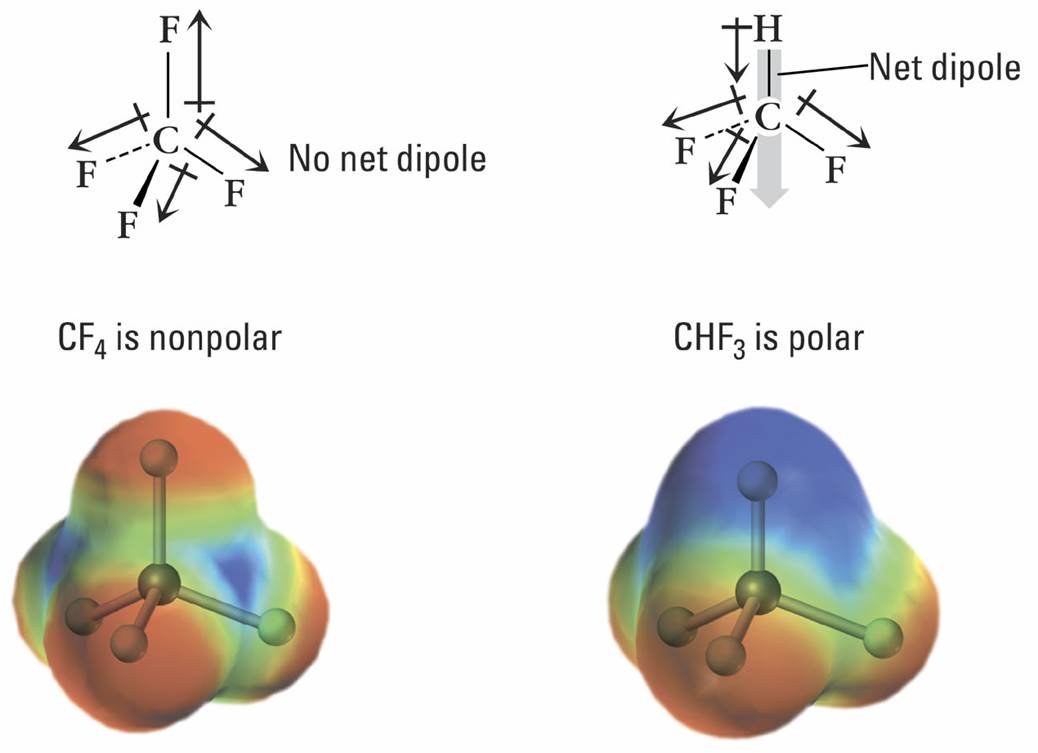
As you can see in the above image, an unbalanced distribution of electrons causes the formation of a polar molecule, whereas an even distribution of electrons between the molecule's atoms causes a molecule to be non-polar.
An important aspect to be taken into consideration when talking about the properties of non-polar molecules is the fact that these molecules only exhibit London Dispersion Forces, the weakest of all the intermolecular forces.
The strenght of intermolecular forces, which are forces that appear between molecules, relates to a lot of physical properties, like melting point, boling point, vapor pressure, surface tension, solubility, and so on.
At room temperature, most non-polar molecules are insoluble in water because water is a polar solvent (the "like dissolves like" rule). Such molecules form hydrophobic, or water-fearing, compounds.
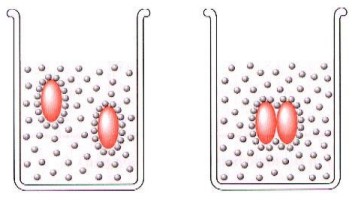
The above image illustrates what happens when a non-polar solute is placed in water, exhibiting the hydrophobic behaviour of non-polar molecules. Therefore, non-polar compounds are most apt to be soluble in non-polar solvents.
When compared to a polar molecule that has a similar molar mass, a non-polar molecule will generally have a lower boiling point and a lower surface tension, due to the weakness of the intermolecular forces it forms.
Non-polar molecules form compounds that have higher vapor pressures than those formed by polar molecules, since less energy will be needed to make them go from liquid to vapor (a higher vapor pressure means a more rapid evaporation).
Nonpolar molecules are still going to experience london dispersion forces (the weakest of the intermolecular forces present in all molecules.)
The strength of the london dispersion forces depend on the size (length if chain) of the molecule.
If a molecule has very strong intermolecular forces then....
- That molecule will have a higher boiling point.
- That molecule will have a higher viscosity.
- That molecule will have a lower vapor pressure.
If a molecule has very weak intermolecular forces then..
- That molecule will have a lower boiling point.
- That molecule will have a lower viscosity.
- That molecule will have a higher vapor pressure.
This comparison works between nonpolar-nonpolar, polar-polar, or even nonpolar-polar molecules.


