How do we use bromine water to distinguish among saturated hydrocarbons, unsaturated hydrocarbons, and phenols?
1 Answer
If the colour changes instantaneously and there is no precipitate, you have an addition reaction.
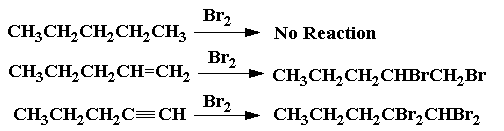
The bromine test is a qualitative test for the presence of alkenes, alkynes, and phenols.
A solution of bromine has a deep reddish-brown colour.
If you add the solution dropwise to an alkene or alkyne, the colour disappears almost immediately, because the product is colourless.
 www.chem.uiuc.edu
www.chem.uiuc.edu
These are addition reactions.
Alkanes give no visible reaction under these conditions. The reddish-brown colour does not disappear.
If a white precipitate forms along with the decolouration, a phenol is present.
Phenols are so reactive that they react instantaneously with bromine to form a white precipitate. For example, phenol forms a precipitate of 2,4,6-tribromophenol.
 images.tutorvista.com
images.tutorvista.com
This is an electrophilic aromatic substitution reaction.
Here's a video on the bromine test for unsaturation.

