Question #5c847
1 Answer
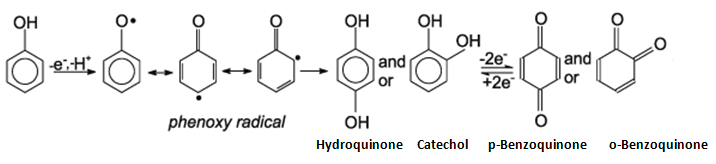
It's hard to say what you mean, but it looks like you're saying the oxidation of phenol into a quinone?
That would be:
 http://s3.mnimgs.com/
http://s3.mnimgs.com/
Based on the behavior of oxygen and the sequence above, my best educated guess for the mechanism is one with oxygen, where heat or light catalysis excites it into its singlet state:
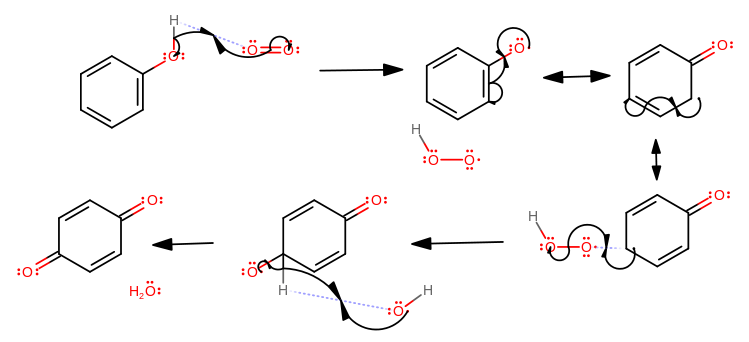
In essence, a radical resonance mechanism. The intermediate would be a phenoxy radical. The ortho and para resonance structures have similar stabilities, but as you may have learned already, the meta is less stable than both. So you can get a mixture of quinones, resulting from the ortho and para phenoxy radical intermediates.
You can see that if you start from step three, ignoring the third product and imitating the arrows in steps four and five, you can also get:
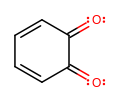
The two products would be called p-benzoquinone and o-benzoquinone, respectively.

