What is the difference between atoms and ions; and covalent compounds and ionic compounds?
1 Answer
Refer to the explanation.
Explanation:
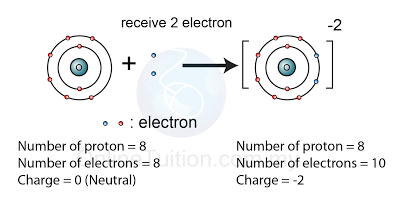
An atom is the smallest particle of an element that has the properties of that element. An ion is an atom that has gained or lost one or more electrons and developed a positive or negative charge. For example, a neutral atom of the element oxygen is represented by the symbol O. However, the oxide ion,
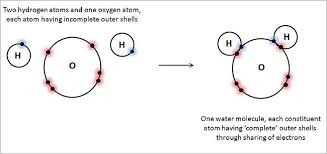
A covalent compound is one in which the atoms are covalently bonded by the sharing of valence electrons. The smallest unit of a covalent compound that has the properties of that compound is a molecule. For example, water is a covalent compound formed when oxygen and hydrogen atoms share valence electrons. One molecule of water is represented by the chemical formula
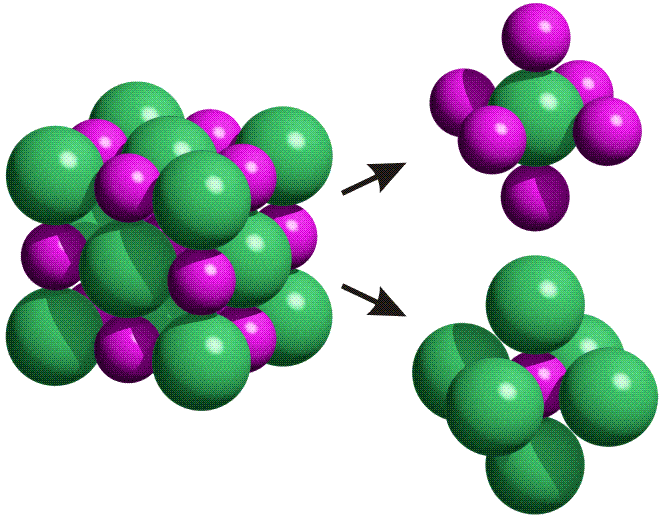
An ionic compound is composed of positive and negative ions bonded by an electrostatic force between the oppositely charged ions, which forms the ionic bond. Ionic compounds do not form molecules. Instead they form crystal lattices, which can be composed of thousands or even billions or more of the positive and negative ions, depending on the size of the crystal.
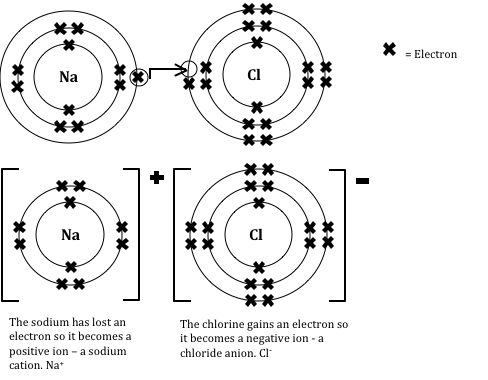
Because of this, the chemical formula for an ionic compound shows the simplest whole-number ratio of ions in the compound, and is called a formula unit. Take, for example, common table salt, with the formula unit NaCl. It is an ionic compound because when it forms, a sodium atom gives up an electron to a chlorine atom, forming a positively charged sodium ion,
The NaCl crystal lattice is composed of alternating sodium and chloride ions. In the diagram below, sodium ions are shown in purple, and chloride ions are shown in green. Notice that each chloride ion in the lattice is surrounded by six sodium ions, and each sodium ion is surrounded by six chloride ions.