Question #1e908
1 Answer
The exchange ion will be
By adding salt to the buffer you're essentially performing the protein elution. The negatively charged proteins that were binded to the positively charged stationary phase particles will now be replaced by the chloride ions.
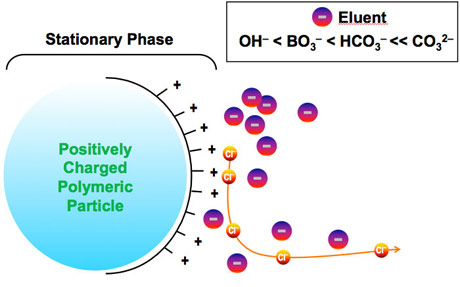
In the initial phase, equilibrium is reached when negatively charged particles, which I assume are chloride ions as well, are binded to the stationary phase particles.
A protein's net surface charge is pH-dependent, i.e. it is determined by the difference between its isoelectric point and the pH of the solution. The concept is pretty basic - at pH values higher than a protein's isoelectric point, the protein will develop an overall negative charge.
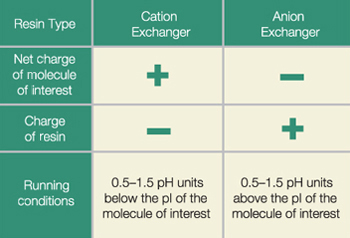
This will allow the protein to be separated and retained by the positively charged stationary phase particles.
The now binded proteins are eluted by changing the concentration of the buffer; in your case, the ionic strength of the buffer is changed by the addition of potassium chloride.
Once this happens, the retained negatively charged proteins will be eluted in order of their respective negative charges and replaced by chloride ions once again, the ones with weaker negative charges being replaced first.
Here's how the basic process looks like
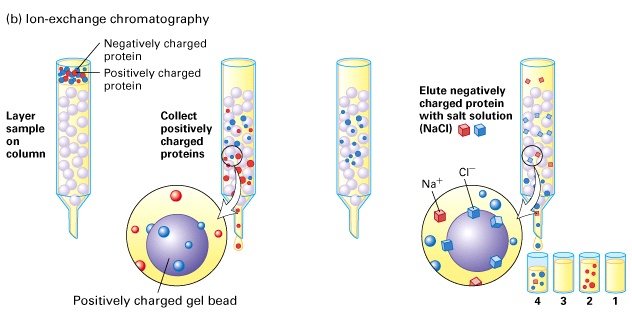
The positively charged proteins will be collected first, since no attraction between them and the stationary phase particles exists; at the same time, the negatively charged proteins will be separated and retained.
Once this happens, elution takes places with the addition of salt - the proteins of interest are replaced by chloride ions,

