Question #c1e01
1 Answer
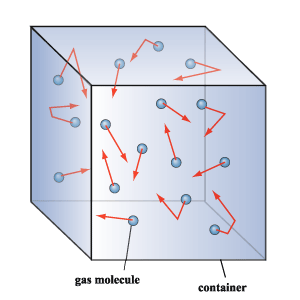
Gas pressure is caused by the collission of gas particles with the walls of a container.
 http://www.daviddarling.info/encyclopedia/G/gas.html
http://www.daviddarling.info/encyclopedia/G/gas.html
Because gas molecules have little to no interaction with each other, they move randomly in an enclosed container. When a gas molecules collides with the walls of the container, it exerts a force on the wall.
This is true for all the molecules that come into contact with the walls of the container - each of them will collide with the wall at different speeds and angles, which means that the force they exert on the wall will vary as well.
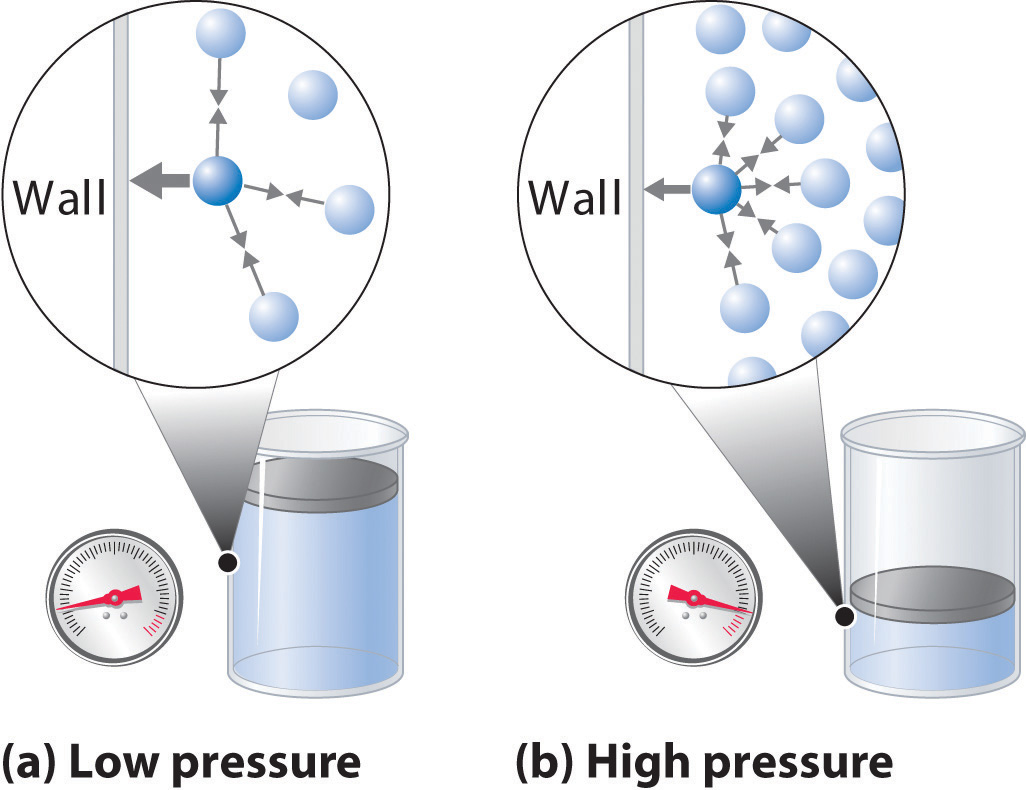
Gas pressure is seen as the average force exerted by the gas molecules per unit area.
That's why temperature and pressure have a proportional relationship when volume and number of moles are kept constant (see Gay Lussac's law) - the faster the molecules move, the more energetic their collission with the walls will be
 http://2012books.lardbucket.org/books/principles-of-general-chemistry-v1.0/s14-08-the-behavior-of-real-gases.html
http://2012books.lardbucket.org/books/principles-of-general-chemistry-v1.0/s14-08-the-behavior-of-real-gases.html

