Question #fe76a
2 Answers
The slope represents
The starting curve for your double reciprocal plot is actually the Michaelis-Menten Plot, which looks like this
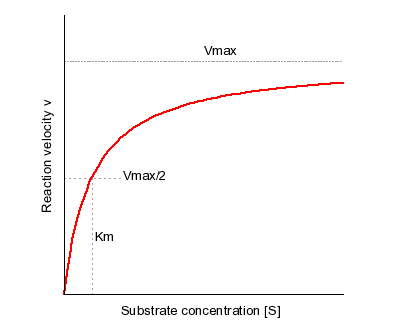
The above plot shows the rate of an enzyme-catalyzed reaction,
The equation plotted looks like this
Now, you need to get from
Now, to get to slope, think of the
This plot is called the Lineweaver-Burk plot and it looks like this
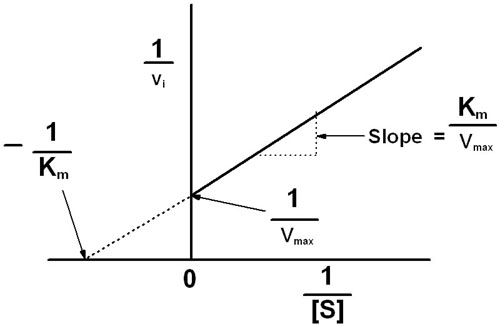
The slope is equal to
A double reciprocal plot is used for analyzing enzyme kinetics.
It is a useful method for analyzing the Michaelis-Menten equation:
where
Taking the reciprocal gives
The equation is of the form
where
A plot of

The
The
And the slope


