The easiest way to go about solving solubility problems is to examine the solubility rules
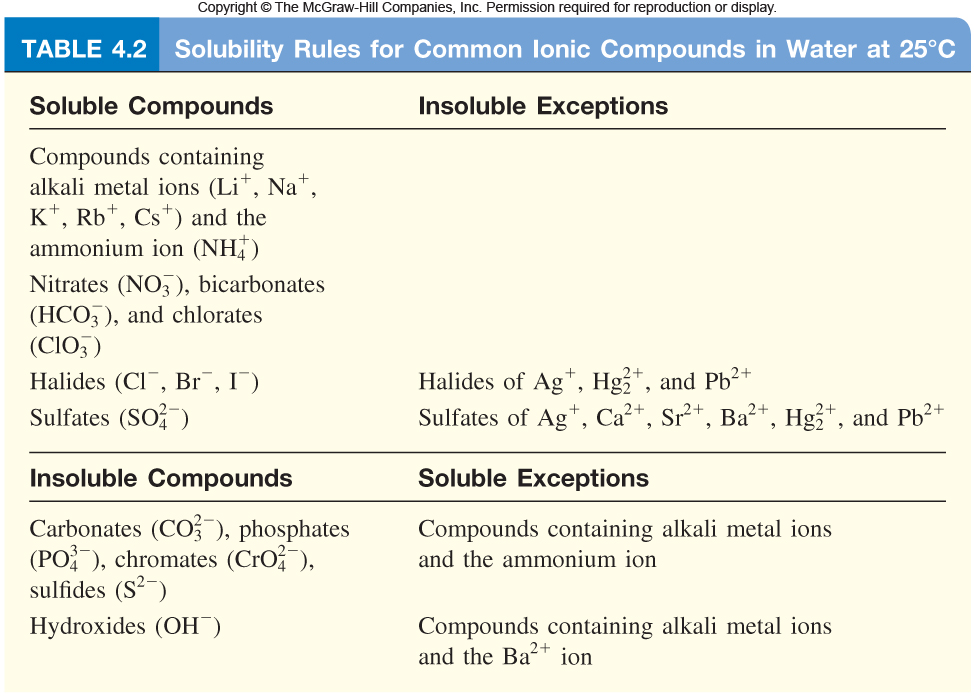
So, starting with mercury (I) chloride, or #Hg_2Cl_2#. As you can see, you're dealing with a halide or, more specifically, with a chloride. Notice that all halides are soluble with the exception of those formed with three cations, including #Hg^(2+)#.
As a result, mercury (I) chloride will be insoluble in water.
Use the same approach for all the compounds listed.
Sodium sulfide, or #Na_2S#, is soluble in water because all compounds that contain the sodium cation, #Na^(+)#, are soluble in water. The same is true for compounds that contain the ammonium ion, #NH_4^(+)#, so ammonium phosphate, #(NH_4)_3PO_4# is soluble in water.
Cadmium carbonate, #CdCO_3#, and lead (II) sulfate, #PbSO_4#, are both insoluble in water because they do no represent soluble exceptions for compounds formed with the carbonate, #CO_3^(2-)#, and phosphate, #PO_4^(3-)# anions.


