What is the electron configuration of #"Pt"^(2+)"#, according to the Madelung rule?
1 Answer
The electron configuration of
The energy of an electron in an atom increases with increasing value of
Within an energy level, as defined by
The sub - levels in order of increasing energy (for the same
The Madelung Rule states that the energy of an electron depends on the value of
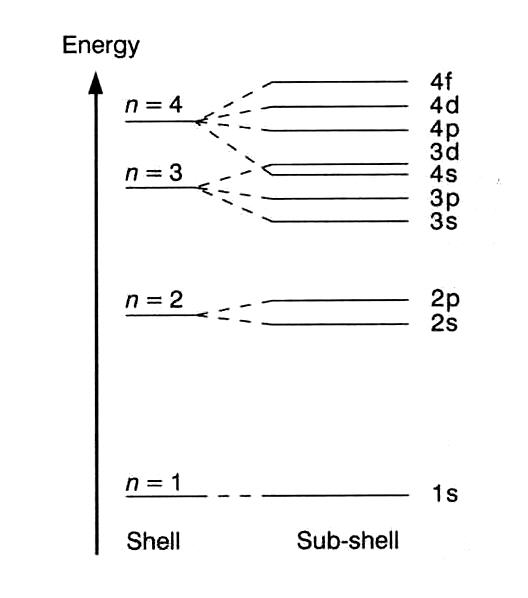
You can see that in the diagram, the
For
For
The problem lies in the fact that this diagram does not apply to all atoms nor should we expect it to.
After calcium the rule starts to break down as complex electron interactions take place. These become especially significant as the electron energy levels get closer and closer together for bigger atoms.
For the first transition series the
Again, as just mentioned, the 4s-3d interactions are more complex than at first glance.
For instance, they are why iron (
Let's apply the Madelung Rule to the examples in your question:
#[Xe]6s^(2)4f^(14)5d^(8)#
For
For
For
When 2 sub - shells have the same value of
So the Madelung Rule/Aufbau principle would predict that configuration. You can apply that reasoning to
The correct configuration according to "Cotton and Wilkinson" is:
#color(green)([Xe]4f^(14)5d^(9)6s^(1))#
This is not in accordance with the Madelung Rule/Aufbau Principle for the reasons already stated.
So losing the
#color(blue)([Xe]4f^(14)5d^(8))#
The Madelung Rule is a rule and not a law so is not applicable in all cases.


