Question #efd75
1 Answer
SIlicon dioxide's empirical formula is
Silicon dioxide forms a giant covalent network very similar to that of diamond, the only difference being the fact that silicon and oxygen atoms alternate in the structure (unlike the structure of diamond, in which you get only carbon atoms).
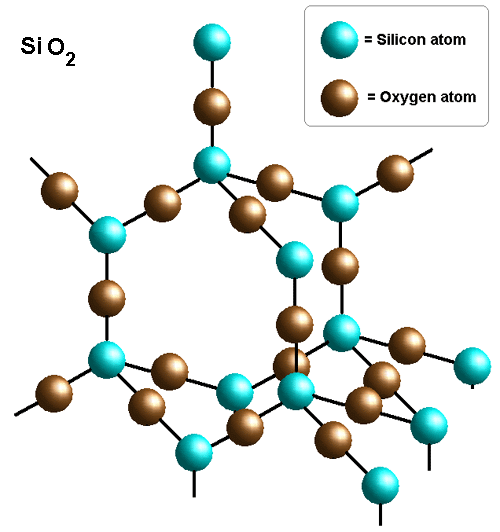
The empirical formula will be determine by the smallest ratio of atoms that exist in that structure. Since you get 2 oxygen atoms for every 1 silicon atom, the empirical formula will be
 )
)
 )
)


