Question #fcf72
1 Answer
Before doing actual calculations, you need the average bond enthalpies for the type of bonds that can be found in your reactants, ethanol and oxygen, and products, carbon dioxide and water.
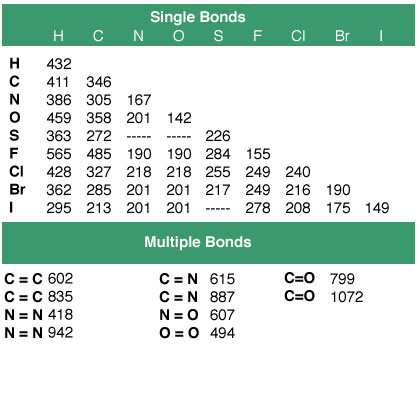
The basic idea is that you can calculate the change in enthalpy for a reaction by using the energy needed to break the bonds of the reactants and the energy given off when the bonds of the products are formed.
The equation looks like this
At a molecular level, ethanol's combustion reaction looks like this
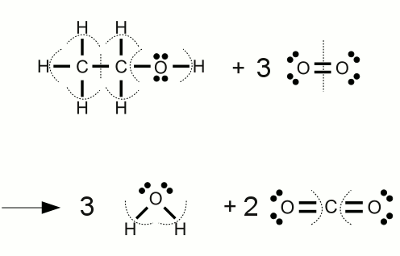
So, you need to break
- 5
#"C-H"# bonds; - 1
#"C-O"# single bond; - 1
#"O-H"# bond; - 3
#"O=O"# double bonds; - 1
#"C-C"# single bond.
And form
- 6
#"O-H"# bonds; - 4
#"C=O"# double bonds.
As a result, the change in enthalpy for the combustion of ethanol is
The minus sign indicates that the reaction is exothermic.

