Question #2486b
1 Answer
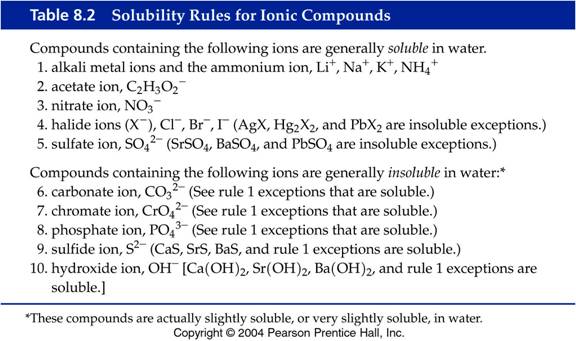
You can determine solubility by using solubility rules like those below.
You indicate the solubility of a compound by writing either the symbol (aq) or (s) after it. If it is soluble in water, the symbol (aq) is used, indicating that the substance dissolves in water, forming an aqueous solution. If a compound doesn't dissolve in water it is insoluble and will be a solid in water, and you use the symbol (s) to indicate this.
Now let's go back to your question about the solubility of ammonium sulfide
Check the solubility rules concerning compounds containing sulfide ions,
Now lets look at another compound, such as barium sulfate,
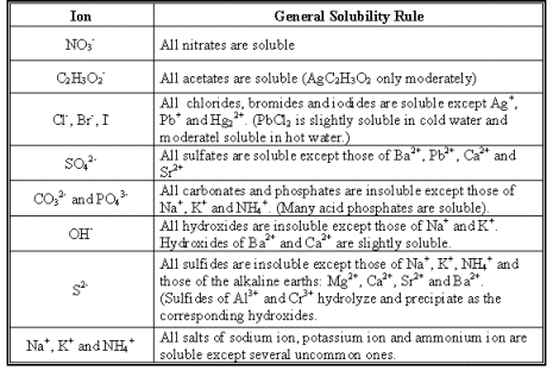
Here is another solubility table.