What is the formula for aluminum oxide? What is the balanced equation for the formation of aluminum oxide?
1 Answer
Apr 30, 2015
Aluminum ions have a 3+ charge (
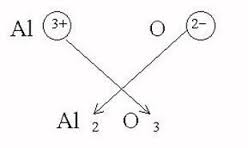
The balanced chemical equation is:
According to the equation, 4 moles of Al react with 3 moles of oxygen to produce 2 moles of aluminum oxide.

