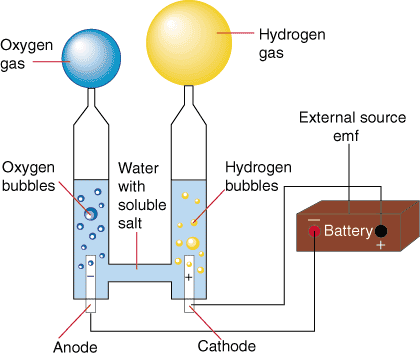
Describe the electrolytic cell where electrolysis of water is performed. What are the signs of the cathode and anode?
1 Answer
The cathode is negatively charged and the anode is positively charged. The positive part of the compound will be attracted to the cathode, and the negative part of the compound will be attracted to anode (opposites attract). The diagram below depicts the electrolysis of water. The electrical current causes the water molecules to dissociate into hydrogen cations and oxygen anions.
Hydrogen cations collect at the negatively charged cathode, forming hydrogen gas from the gain of electrons from the water, and oxygen anions collect at the anode forming oxygen gas by the loss of electrons. One mole of water produces one mole of hydrogen gas and 1/2 mole of oxygen gas.
Reference:
http://www.instructables.com/id/Separate-Hydrogen-and-Oxygen-from-Water-Through-El/