Question #16b9b
1 Answer
Ernest Rutherford's Experiment!
Explanation:
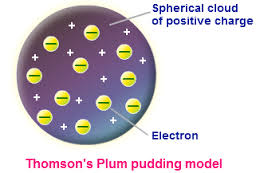
Rutherford actually was the one to discover protons. Previously, people had accepted something like a "plum-pudding model" of an atom, where electrons were evenly distributed throughout an atom (like plums) and the rest of the space was occupied by a "pudding-like" positively charged substance. Rutherford decided that he was going to test out whether or not this was true.
Rutherford took a piece of extremely thin solid gold (perhaps one or two atoms wide). Through this, he ran electromagnetic radiation. If the plum-pudding model was supported by the data, then the data would show that all of the particles would pass through the gold foil. However, this was not the case.
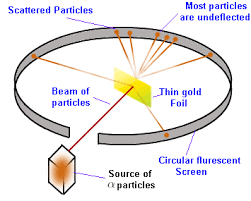
Instead, Rutherford found that what actually happened was that many of the electromagnetic beams were reflected backward, some even directly at where they were fired! This forced Rutherford to create a new model for the atom, which is close to what we go off of today.
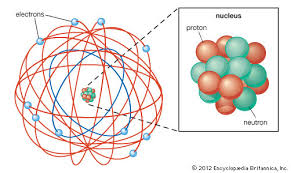
The model had a small, positively charged nucleus in the center of the atom, and negatively particles would be found "racing" around the outside of the model, near the outside of the atom. The discovery of the small nucleus in the middle of the atom is what led to the proton being discovered.
I hope that helps!

