Question #32ecf
1 Answer
Whitewash is made by mixing quicklime, which is another name for calcium oxide,
Calcium oxide is obtained from the thermal decomposition reaction of limestone, which is another name used for calcium carbonate,
When calcium oxide is mixed, or slacked, with water, you get slacked lime, which is calcium hydroxide.
Calcium hydroxide is one of the main ingreadients in whitewash, along with chalk, or calcium carbonate,
Calcium hydroxide is converted into calcium carbonate by a process called carbonation
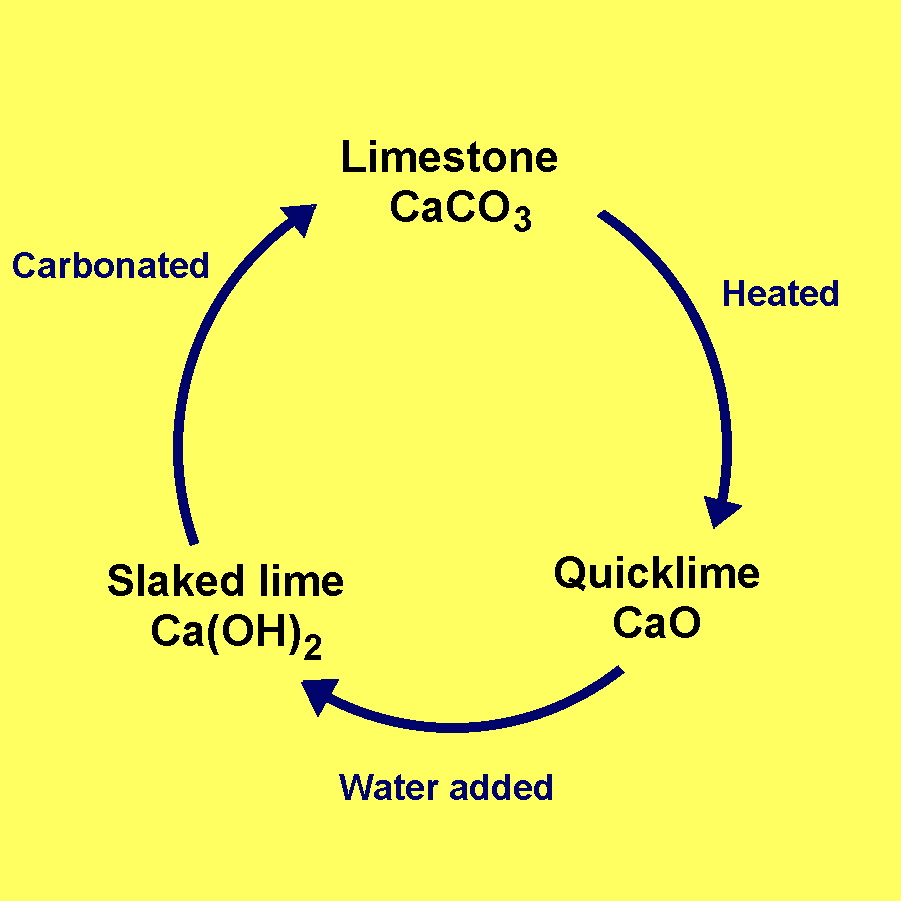
So, as a conclusion, whitewash is a solution that cxontains calcium hydroxide (slacked lime) and calcium carbonate (chalk).

