Question #f3172
1 Answer
The color of the solution changes because of the presence of the iron (II) ion,
Here's how the balanced chemical equation for this single replacement reaction looks like
Iron is more reactive than copper, so it will displace the copper (II) ions,
The color of the initial solution is attributed to the presence of the
On the other hand, the color of the final solution can be attributed to the presence of the
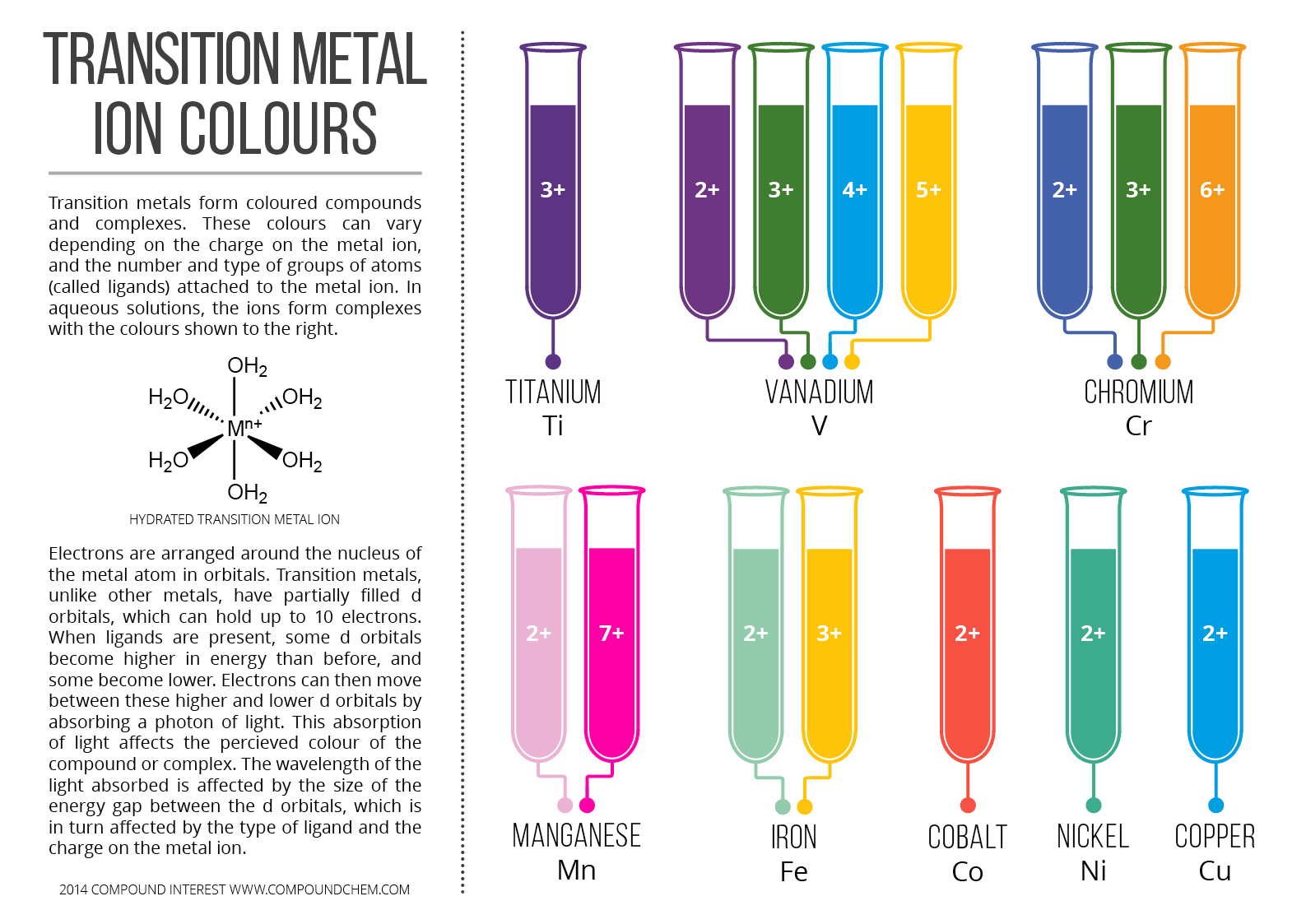
You can actually observe two changes that take place with this reaction
-
the color of the solution turns from blue to light green
-
a brown coating is deposited on the nail
The brown coating you see on the nail is actually the copper that was displaced from the solution by the iron (II) ions.
You can think of this as a redox reaction as well. Iron metal is being oxidized and becomes

