Question #32d5f
1 Answer
The relationship between moles and grams involves the concept of molar mass, which represents the exact mass in grams of 1 mole of a substance.
Remember that 1 mole of a substance is defined as exactly
Depending on what substance you're dealing with, the mass of
This is where molar mass comes into play. If you know the mass of 1 mole of a substance, you can use the following relationship
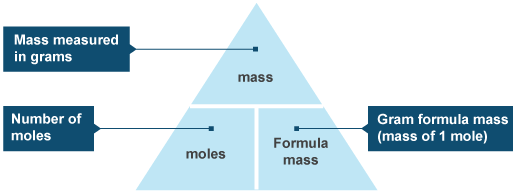
Simply put, you can go from moles to grams and vice versa by using the mass of 1 mole of that substance, i.e its molar mass.
For example, the molar mass of carbon is 12.011 g/mol. This means that 1 mole of carbon, or
So, if you have 2 moles of carbon, you'll have
Likewise, if you have 54 g of carbon, you'll have

