Question #dfce4
1 Answer
Hückel's rule applies only to aromatic compounds.
Antiaromatic compounds differ from aromatic compounds in that they
- are highly unstable
- are highly reactive
- show a paramagnetic ring current in NMR
Hückel first worked out the quantum mechanics of his rule in 1931, and William von Doering formulated it in 1951 as the 4n+2 rule.
Ronald Breslow proposed the concept of antiaromaticity in 1967.
To be antiaromatic, a molecule must:
- be cyclic
- be planar
- have a cyclic, conjugated π system
- have 4n π-electrons
Examples of antiaromatic compounds are pentalene (A), biphenylene (B), cyclopentadienyl cation (C).

Many compounds become nonplanar and break the π interactions in order to avoid becoming antiaromatic.
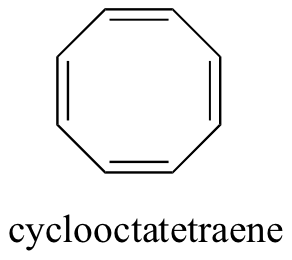
For example, planar cyclooctatatraene is expected to be antiaromatic.
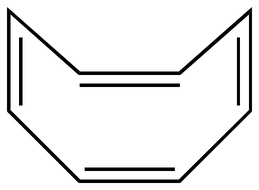
Instead, the molecule puckers to form a tub shape to avoid the instability of being antiaromatic.
NMR Ring Currents
Antiaromatic compounds show a paramagnetic ring current.
This causes the outer protons to be shielded and the inner protons to be deshielded.
[12]annulene has 3 protons both inside and 9 outside the ring.

The chemical shift for the inner protons is δ 5.91 and that for the protons outside the ring is δ 7.86, compared to δ ~6.5 for ordinary alkenes.

In contrast, the inner protons of aromatic [14]annulene appear at δ 0.0 and the outer protons appear at δ 7.6.

