Question #054d9
1 Answer
Because the atmospheric pressure at higher altitudes is lower than at sea level.
Water, or any liquid for that matter, boils when its vapor pressure equals the external pressure above the liquid.
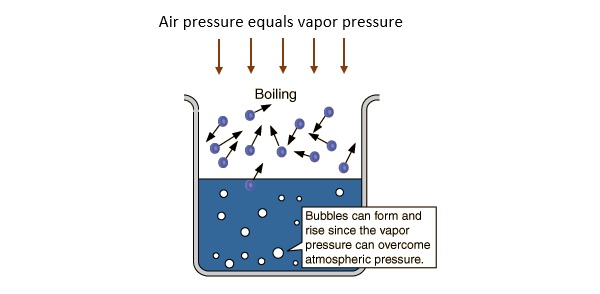
In water's case, you need to heat it to
As elevation increases, atmospheric pressure decreases. This means that you'll need to supply less heat to the liquid water in order to get its vapor pressure to equal the atmospheric pressure.
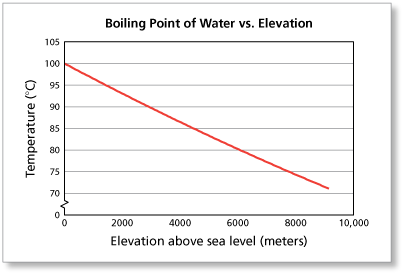
For example, on top of Mount Everest, the atmospheric pressure is approximately 0.34 atm. When you heat water at that altitude, you'll find that it'll start to boil at around
That happens because water's vapor pressure reaches the value of the atmoshperic pressure faster, i.e. without requiring as much heat as it would at sea level.
Another answer on this topic:
http://socratic.org/questions/how-do-boiling-points-change-at-high-altitudes?source=search

