What functional groups are in saccharin?
1 Answer
There is only one functional group in saccharine — a carboxylic sulfonimide (highlighted with color in the image below). The chemical name of saccharin is benzoic sulfimide.
The structure of saccharin is
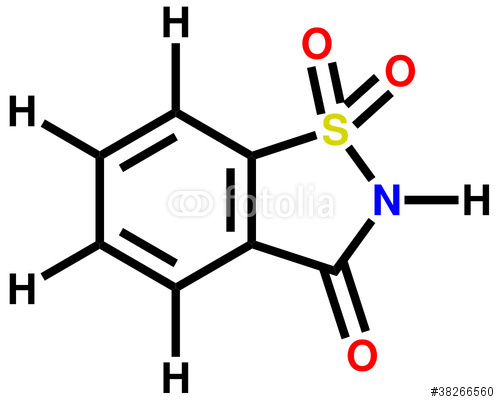
The
That makes it an amide:
On the other side, the
That makes it a sulfonamide:
If there had been two carbonyl groups on either side, we would have an imide:
An imide is the nitrogen analogue of an anhydride.
If there had been two sulfonyl groups on either side, we would have a sulfonimide:
AS it is, we have a carbonyl group on one side and a sulfonyl group on the other:
We would have to call this half-and-half group a carboxylic sulfonimide.


