Two aqueous solutions react to form a precipitate. What kind of reaction is this?
1 Answer
When two aqueous solutions react to form a precipitate, a chemical reaction called a double replacement (displacement) reaction has occurred. The general equation for a double replacement reaction is AX + BY
The reason that a precipitate forms is that one of the products is insoluble in water. We can determine what the precipitate is by using solubility rules.
For example,
Silver nitrate
To determine which product is the precipitate, you need to check the solubility rules to determine which product is insoluble.
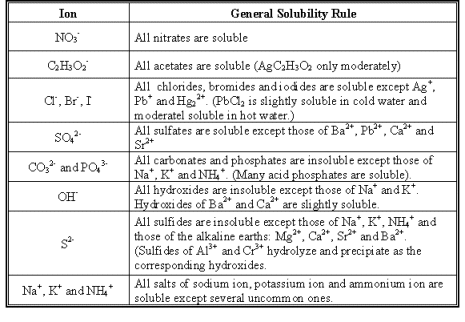
As you can see, all nitrates are soluble, and silver chloride is insoluble. Therefore, silver chloride is the precipitate.
So, now you can finish writing the equation.

