What is the pattern of filling in the p orbitals?
1 Answer
Jun 7, 2015
Each p orbital must have a single electron with parallel spin before adding another electron. The second electron, with opposite spin, is added to each orbital to complete the p sublevel. In reality, it doesn't matter which axis a p orbital is in. They all have equal energy and any one of them can be filled first. However, if the
The diagram below shows the orbital diagrams for the elements in the second period of the periodic table.
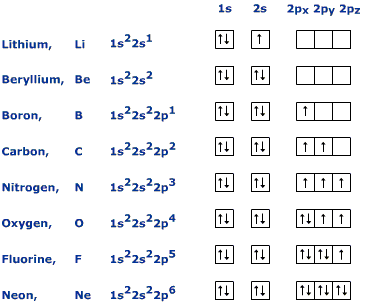 http://apocketmerlin.tumblr.com/post/20075430215/electronic-structure-the-basics
http://apocketmerlin.tumblr.com/post/20075430215/electronic-structure-the-basics

