Question #3fc1b
1 Answer
Jun 8, 2015
Ammonium nitrate,
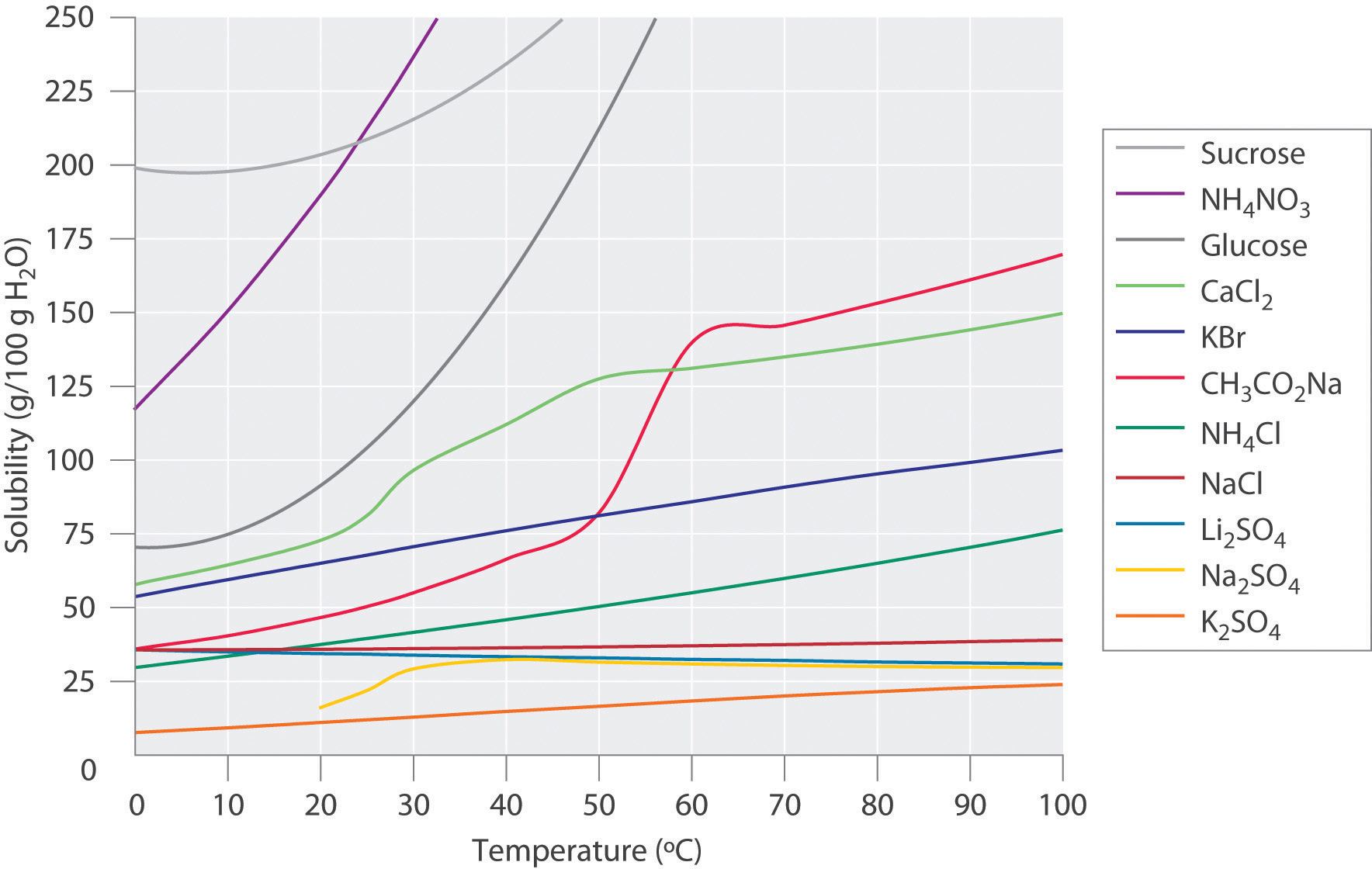
In this solubility diagram, ammonium nitrate is represented by a
At
You can see a more detailed solubility curve for
Ammonium nitrate,

In this solubility diagram, ammonium nitrate is represented by a
At
You can see a more detailed solubility curve for