Question #3447f
1 Answer
This reaction produces hydroxyapatite and hydroxide anions.
Explanation:
When calcium hydroxide,
The balanced chemica lequation for this reaction looks like this
The complete ionic equation looks like this
If you remove spectator ions, which are ions present on both sides of the equation, you get the net ionic equation
This reaction is actually used to remove phosphate ions from various solutions.
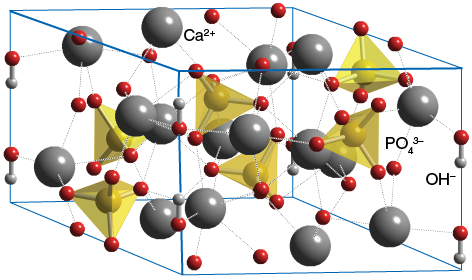
Here's how the structure of hydroxyapatite looks like: