A good leaving group has to be able to part with its electrons easily enough, so typically, it must be a strong acid or weak base relative to other substituents on the same molecule. It helps to know the pKa of what would be leaving.
Let's say you had a mechanism where you are trying to do an E2 reaction to make an #-OH# (hydroxyl) group leave. Maybe you have this compound on hand, sec-butanol (or 2-butanol). Let's say that at the stereocenter, the #-OH# group was in front (solid wedge), with a proton in the back (hash).
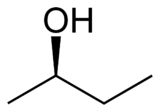
If we were to call this #S# or #R# (you've done that by now, right?), the ranking would go, from #1# to #4#, #-OH#, #-CH_2CH_3#, #-CH_3#, #-H#. So, it would be #R#.
Now, notice how if you do an E2 reaction, you've got two possibilities for a product: a cis/trans-alkene, or a terminal alkene. According to Zaitsev's Rule, the adjacent carbon with fewer protons is more willing to give up a proton for the E2 reaction.
If we wanted to facilitate specifically E2, I would suggest a bulky nucleophile like tert-butoxide (e.g. #t"-"BuO^(-)K^(+)#), and heating the reaction (#Delta#). It is really unlikely then, that it'd do SN2, based on its steric hindrance and the high temperature conditions.
Also, normally, #-OH# isn't that great of a leaving group. You can make it better by protonating it, let's say with #HCl#.
#CH_3(COH_2^(+))CH_2CH_3 stackrel(Delta)(stackrel(t"-"BuO^(-)K^(+))(rightleftharpoons)) overbrace(H_2C=CHCH_2CH_3)^"terminal" + overbrace(H_3C(HC=CH)CH_3)^"cis/trans"#
It would grab the proton either in the back on carbon-1 or the one in the back on carbon-3. That way it's antiperiplanar with the #-OH_2^(+)# and it's in the proper orientation for the elimination reaction (imagine or draw out a sawhorse projection to see it better).
As a result of all that, the protonated #-OH_2^(+)# makes for a pretty good leaving group (leaving as #H_2O#)! This leaving group has a pKa of #15.7#, relative to everything else, which are all alkyl (#50~60#), much stronger bases than water, so water easily leaves most often.


