Which one is more acidic? Acetone or acetaldehyde? Methyl acetate or acetone?
1 Answer
Here you can see the deprotonation of acetone and acetaldehyde, respectively.
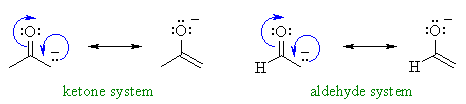
For acetone and acetaldehyde specifically, the explanation is simply that the substituent on acetone is larger than on acetaldehyde (CH3 vs. H).
The larger the substituents attached, the more electron-electron repulsion the deprotonated other carbon has with the carbonyl oxygen (larger substituent, more steric strain from orbital crowding, more repulsion).
More repulsion
→ less stable anion
→ more stable (less acidic) regular molecule
→ higher pKa on [the molecule with the larger substituent] that performs the electron sharing.
Their resonance structures are identical aside from those two substituents as the difference.
Similarly, here you can see the enolate of methyl acetate:
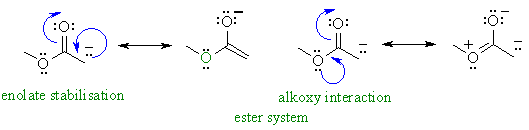
For methyl acetate vs. acetone specifically, besides the substituent size, knowing how it has two competing resonance structures (deprotonated carbon with carbonyl vs. the alkoxyl oxygen with the carbonyl), if we look at the oxygen-oxygen resonance structure, the alkoxyl oxygen stabilizes the carbonyl.
But that leaves the
(
→ anion less stable
→ regular ester is more stable
→ less acidic
→ higher pKa).

