Question #24c67
1 Answer
Jun 27, 2015
That much sodium nitrate contains
Explanation:
Since sodium nitrate is an ionic compound, you can't really say that you're dealing with molecules, but with formula units.
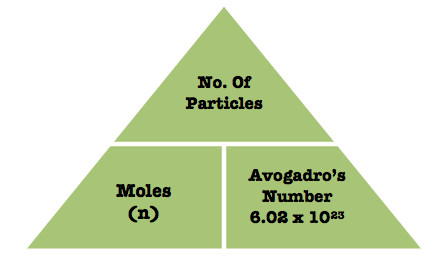
In order to calculate how many formula units a certain mass of a substance contains, you need to do two things
- determine how many moles are present in that many grams by using the substance's molar mass;
- determine how many formula units you have in that many moles by using Avogadro's number.
So, use sodium nitrate's molar mass to determine how many moles are present in 2 g
Avogadro's number tells you that 1 mole of any substance contains exactly
This means that you have

