What is the relation between critical temperature and boiling point or vapor pressure?
1 Answer
Higher
This is easier to see with a phase diagram. Consider the phase diagram of
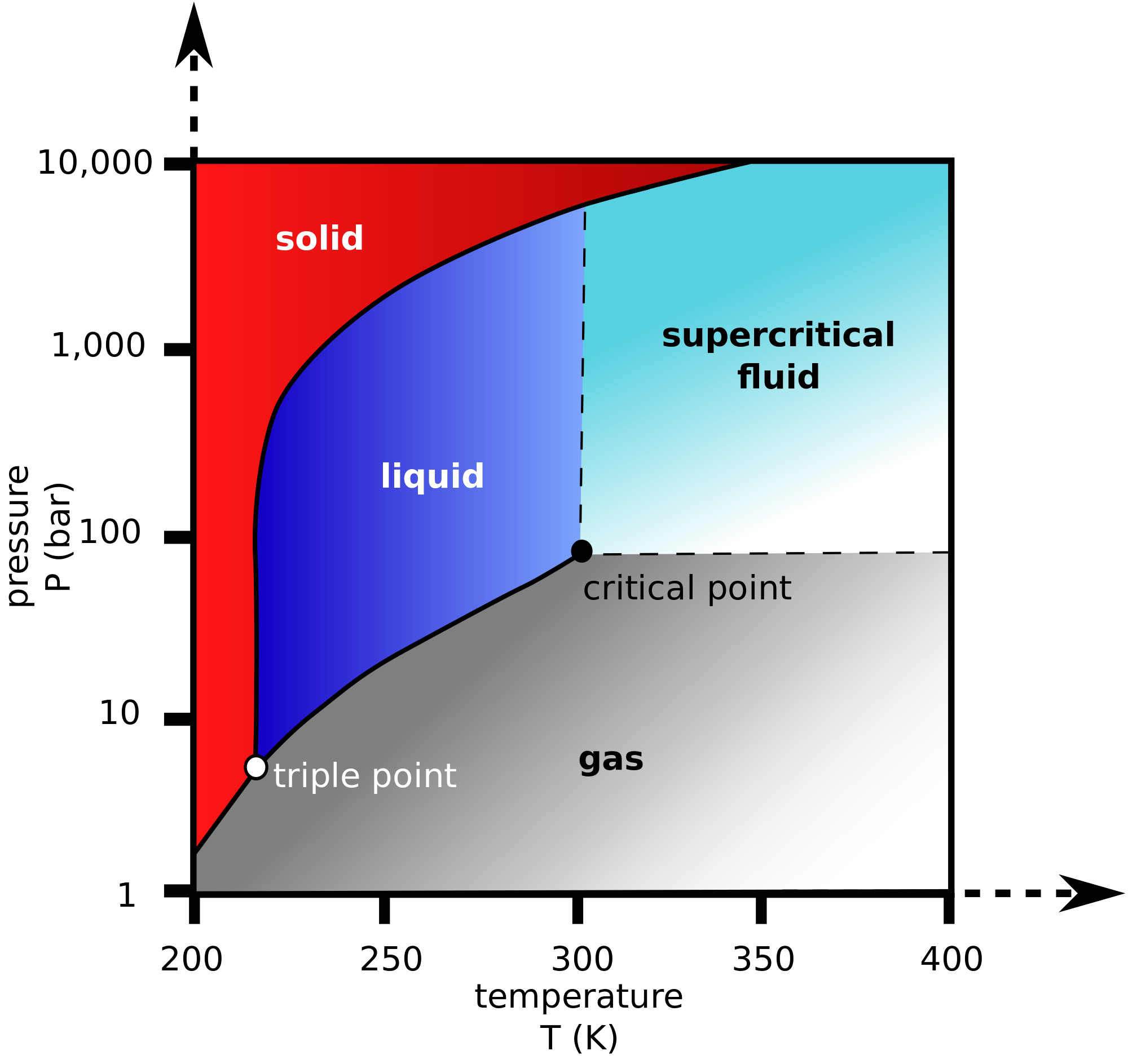
The critical temperature
For
When we consider the substance with the higher vapor pressure, it thus vaporizes more easily, having more vapor in equilibrium with the liquid phase, giving it a lower boiling point.
But going from left to right (i.e. heating), a liquid needs to boil to become a gas. Thus, if boiling is easier, reaching the critical temperature should be easier as well.
Thus (with exceptions in mind),
For example... see the trend here. All data are derived from NIST phase change data.
#"CO"_2# :
#P_(vap) = "64.01 bar"# at#25^@ "C"# ,#T_c ~~ "304 K"#
#T_b = -78.5^@ "C"#
#"CH"_3"Cl"# :
#P_(vap) ~~ "6.5 bar"# at#25^@ "C"# ,#T_c ~~ "416 K"#
#T_b = -24.2^@ "C"#
(vapor pressure estimated, b/c missing data points)
#"CH"_3"CH"_2-"O"-"CH"_2"CH"_3# :
#P_(vap) ~~ "0.6688 bar"# at#25^@ "C"# ,#T_c ~~ "467 K"#
#T_b = 34.6^@ "C"#
#"CH"_3-("C"="O")-"CH"_3# :
#P_(vap) ~~ "0.3078 bar"# at#25^@ "C"# ,#T_c ~~ "508 K"#
#T_b = 56.2^@ "C"#
#"H"_2"O"# :
#P_(vap) ~~ "0.0317 bar"# at#25^@ "C"# ,#T_c ~~ "647 K"#
#T_b = 100.0^@ "C"#
#"I"_2# :
#P_(vap)# #"<<"# #"0.0112 bar"# at#25^@ "C"# ,#T_c ~~ "819 K"#
#T_b = 184.4^@ "C"#

