Question #d7c12
1 Answer
Jul 8, 2015
First, you draw the formula. Then you count the bonds.
Explanation:
The formula of pent-3-en-1-yne is
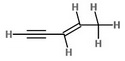
Every C-C and C-H bond has one σ bond.
There are 6 C-H bonds and 4 C-C bonds.
So there are 10 σ bonds.
All the extra bonds are π bonds.
So the C=C double bond has 1 π bond, and the C≡C triple bond has 2 π bonds.
This makes a total of 3 π bonds.
The molecule has 10 σ bonds and 3 π bonds.

