Question #07b6f
1 Answer
The temperature of the water will actually decrease.
Explanation:
Dissolving potassium nitrate,
An endothermic process is characterized by the fact that heat is being absorbed by the reaction.
In your case, this happens because the energy needed to dissociate the potassium cations and the nitrate anions from each other in the solid exceeds the energy released when these ions are hydrated.
The difference in energy will be obtained from the surroundings, in this case from the water. This will result in an overall temperature drop, i.e. the solution will start to cool.
Potassium nitrate's solubility graph looks like this
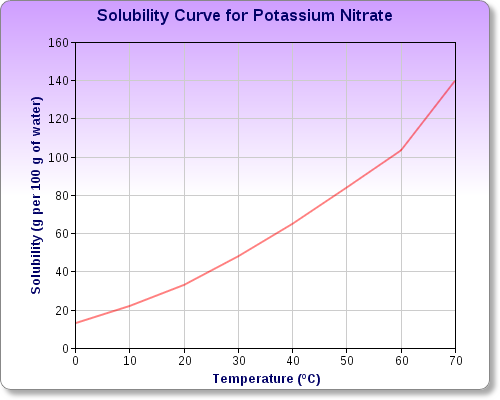 http://www.sciencequiz.net/jcscience/jcchemistry/water_solutions/water_solutions_mcq.htm
http://www.sciencequiz.net/jcscience/jcchemistry/water_solutions/water_solutions_mcq.htm
At
So, depending on how much potassium nitrate you actually dissolve, and what the mass of the water is, you can expect the temperature of the water to drop by anywhere from a few to several degrees Celsius.
Check out this answer for a more detailed explanation on why dissolving potassium nitrate in water is an endothermic process:
http://socratic.org/questions/why-is-dissolving-potassium-nitrate-in-water-an-endothermic-process

