Question #e2a78
1 Answer
Because they are the same chemical compound.
Explanation:
If I understand your question correctly, hydrogen chloride, or
In essence, a hydrogen chloride molecule is formed when a hydrogen atom and a chlorine atom form a covalent bond.
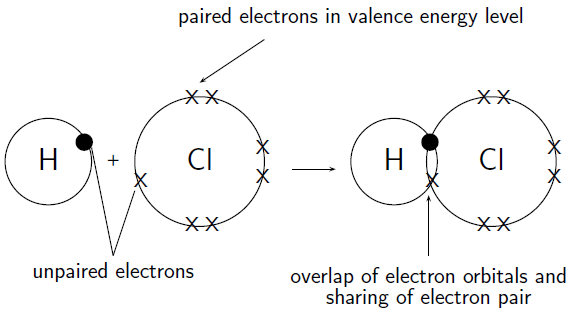
The fact that chlorine is more electronegative than hydrogen ensures that this bond is actually a polar covalent bond, meaning that the bonding electrons spend more time surrounding the chlorine atom than they do the hydrogen atom.
Now, there is a subtle difference between hydrochloric acid and hydrogen chloride
When the molecule is in a gaseous state, it is called hydrogen chloride.
When it is placed in aqueous solution, the compound is called hydrochloric acid.
So basically, the
- in gaseous state
#-># hydrogen chloride; - In aqueous solution
#-># hydrochloric acid.

