Question #9327a
1 Answer
Jul 20, 2015
You start with a wedge-dash structure.
Explanation:
Then you convert the wedge-dash structure to a Newman projection.
The wedge-dash structure for the staggered conformation is shown below.
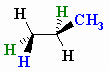
(from www.chem.ucalgary.ca)
You want to convert it to a Newman projection through the
So you must view the molecule along the
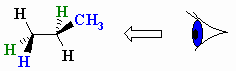
(from www.chem.ucalgary.ca)
The closest bonds make a "Y", so choose a template with the bonds making a "Y" on the front carbon.
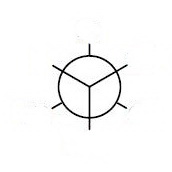
Place the groups onto your template.
- The groups on
#"C-2"# go on the front carbon; the groups on#"C-3"# in back. - The groups with "normal" bonds go on the vertical lines:
#"H"# in front and back. - The groups with wedged bonds go on the left hand side:
#"CH"_3# in front and#"H"# in back. - The groups with dashed bonds go on the left hand side:
#"H"# in front and back.
And you have a Newman projection for propane.
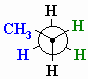
(from www.chem.ucalgary.ca)

