Question #51b1b
1 Answer
Aug 14, 2015
o-Nitrophenol has strong intramolecular hydrogen bonds, but p-nitrophenol has strong intermolecular
Explanation:
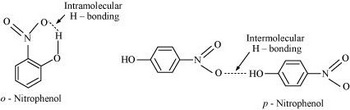
(from www.meritnation.com)
p-Nitrophenol has strong intermolecular hydrogen bonds.
These strong intermolecular forces mean that has a low vapour pressure
In fact, the compound decomposes before it reaches a normal boiling point.
In o-nitrophenol, the hydrogen bonds are associated with the adjacent nitro group.
The intramolecular forces are much weaker than in the para isomer, so the vapour pressure is much higher.
The normal boiling point is till high (216 °C), but the vapour pressure is high enough that o-nitrobenzene is easily steam distilled.

