Question #96750
1 Answer
I am assuming by a table, you want to know different examples, so I will try to briefly describe each, and then give examples.
Explanation:
The number of carbon atoms determines the prefix of the hydrocarbon.
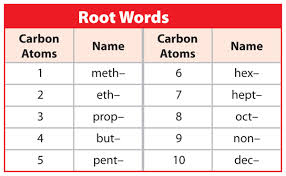
Alkanes are a branch of hydrocarbons with the formula 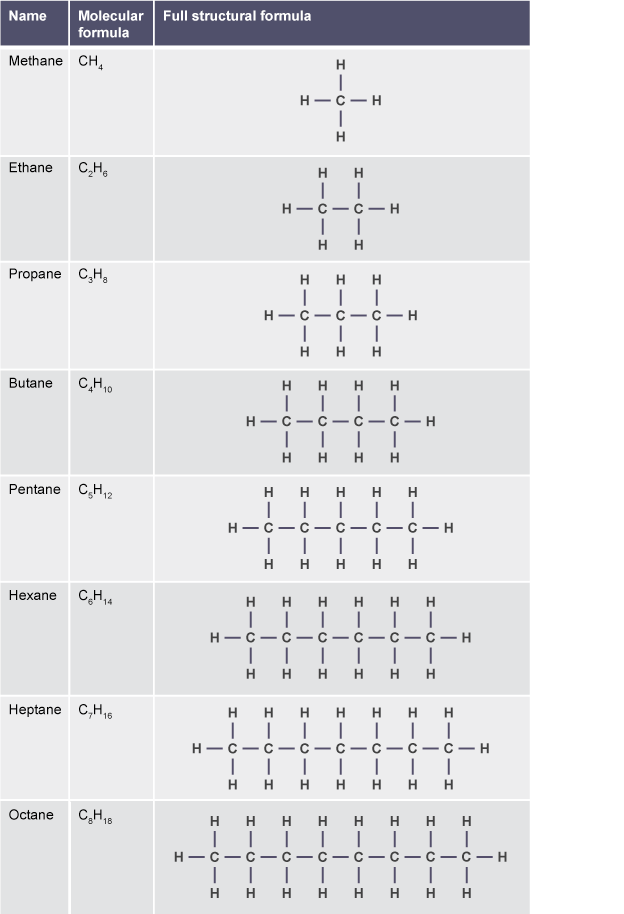
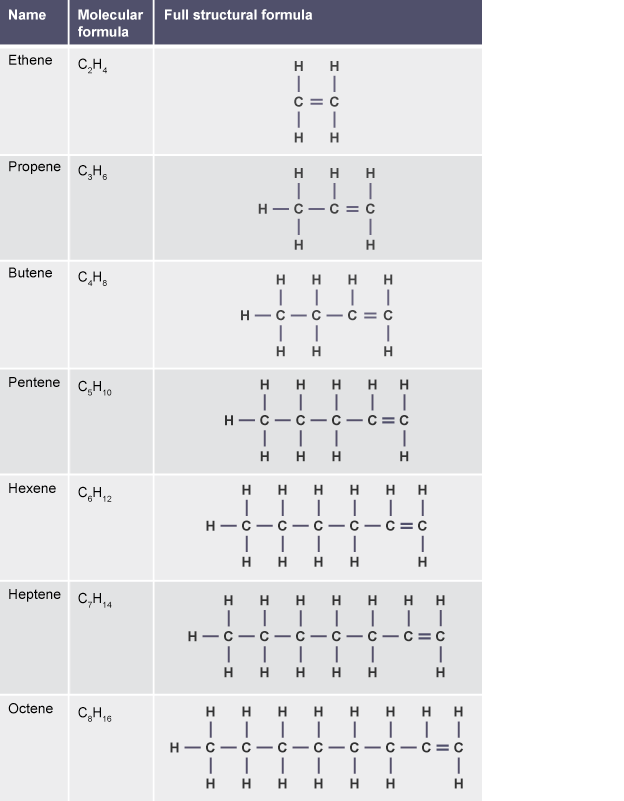
Alkenes have the formula
Finally, alkynes are represented by 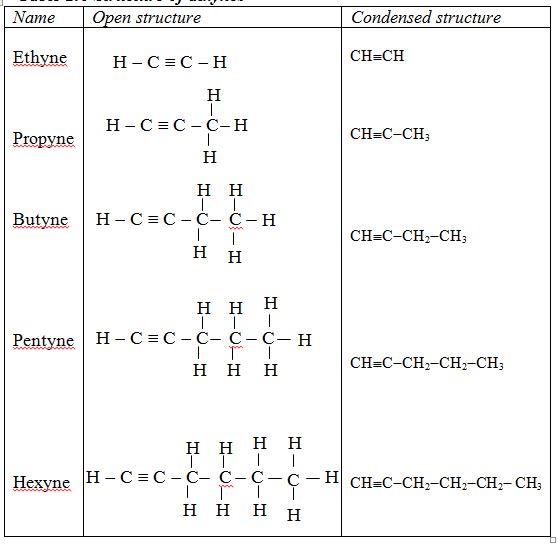
So if you have a hydrocarbons formula, you can use it to find out whether it is an alkane, alkene or alkyne by the ratio of carbon to hydrogen, and you can find the prefix by the number of carbon atoms.
E.g. if you have an unknown substance with the formula
As for the table, I did attempt to create one for you on excel, but after doing all the coding and editing my macro to form chemical formulas, my computer malfunctioned - so I have included a few reliable ones from BBC bitesize and Schule Direct.
I hope this helps, feel free to let me know if you need anymore information, or something explaining better.
