Characterize the IR spectrum of methyl benzoate?
1 Answer
First, let's get the structure down. Methyl, indicating the alkyl group coming off the bottom-right carboxyl oxygen of a benzyl ester group.
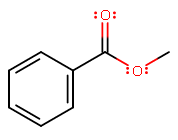
Within this molecule, we have the following relevant bonds:
Notice how a double bond will not easily bend, so we don't really talk about those bending, only stretching.
Within the bonds we've just listed, we have the following resonant frequencies that characteristically coincide with the most prominent vibrational activities of each functional group (Techniques in Organic Chemistry; Mohrig, Hammond, and Schatz):
1620~144"0 cm"^(-1) , medium to weak strength (stretch)
3100~300"0 cm"^(-1) , medium to weak strength (stretch)
900~68"0 cm"^(-1) , strong strength (bend)
1730~171"5 cm"^(-1) , strong strength (stretch)
1300~100"0 cm"^(-1) , strong strength (stretch)
2990~285"0 cm"^(-1) , medium to strong strength (stretch)
1480~1430, 1395~134"0 cm"^(-1) , medium to weak strength (bend)
You can see the spectrum here:
http://sdbs.db.aist.go.jp/
Specifically:
- The aromatic
"C"="C" stretch is the thin weak peak you see near1600 - The aromatic
"C"-"H" bend is the thin strong peak you see near700 and the stretch is the thin, well-defined, weak peaks right above3000 - The ester
"C"="O" conjugated stretch is the strong, slightly wide peak right above1700 - The ester
"C"-"O" stretch is the strong, slightly wide peak you see right below1300 - The alkane
"C"-"H" bends are the thin medium/weak peaks you see near1500~1300 (including the "sidearm" off of the ester peak) while the single thin weak peak near3000 is the stretch.
BONUS POINTS: can you spot the

