Question #57aa3
1 Answer
When two isomeric molecules which have different 3 dimensional shapes in space.
Explanation:
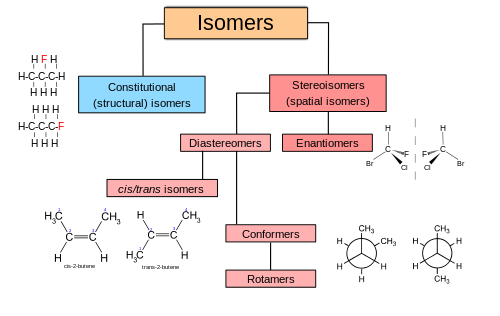
Isomeric molecules are molecules that have the same chemical formula.
Constitutional isomers are molecules which have the same chemical formula but have a different structure.
For example, a long chain hydrocarbon can have an alcohol group on one of many carbon atoms but still keep the same formula.
Stereoisomers have the same formula and structure but have different three dimensional shapes in space.
For example, if you have a double bond in a long chain hydrocarbon, the hydrogen atoms can be on the same side of the chain as each other (cis) forming a kink in the chain, or can be on opposite sides (trans).
Enantiomers are another example of molecules with stereoisomersim, as they have the same formula and bonding but instead are mirror images of each other, thus cannot overlap in anyway to form the same molecule.