Why is cyclopropane unstable?
1 Answer
Sep 24, 2015
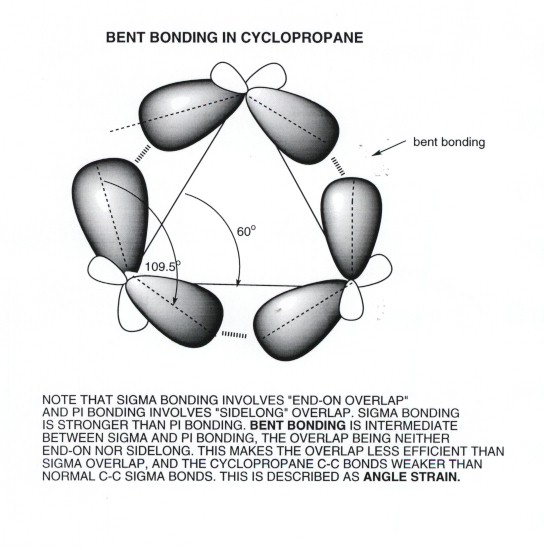
The bonding in cyclopropane is extremely strained. So strained that the orbitals themselves are not aligned in the normal fashion. Some call them "banana" bonds. Instead of aligning colinearly or parallel, the orbital alignment here is bent, creating an in-between stability and strength---forming bonds that are weaker than