Question #2748c
1 Answer
The five aromatic isomers are anisole, benzyl alcohol, and o-, m-, and p-cresol.
Explanation:
Your compound has an "unsaturation value" of 4.
That means it must have four rings and/or double bonds.
I immediately think of a benzene ring, which has one ring and three double bonds.
There are, of course, many other non-aromatic possibilities, but there are too many to include here.
A. Monosubstituted benzene ring
The phenyl ring is
The only possibilities are
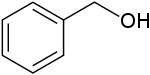
One possibility is anisole

The other possibility is benzyl alcohol
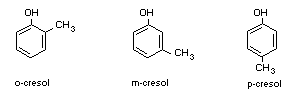
B. Disubstituted benzene ring
A disubstituted benzene is
The other substituents must be
The only possibility is
The compounds are o-, m-, and p-cresol.