Question #585a0
1 Answer
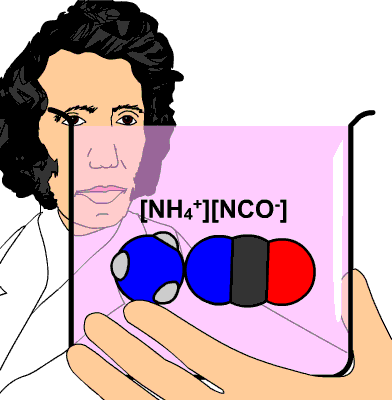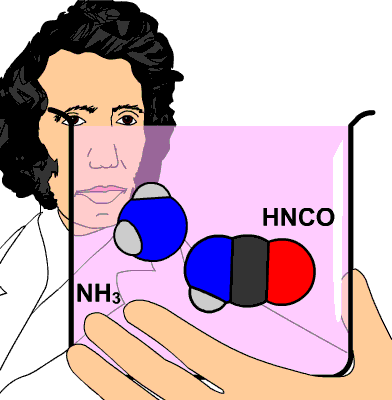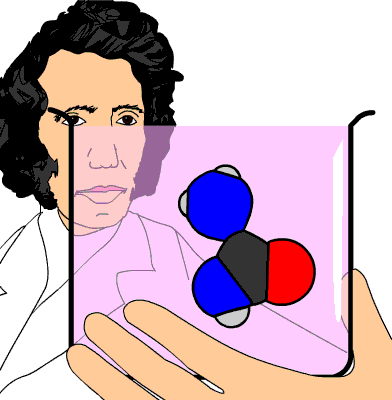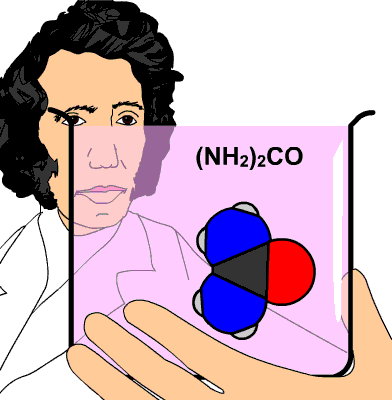
Indeed, the cyanate ion is
Explanation:
The reaction you're describing is called the
The idea here is that you decompose ammonium cyanate,
These two compounds will then react to form urea,
So, how do you know what the formula of the cyanate ion present in the ammonium cyanate is? Look at the chemical formula of urea.
Urea contains
- one carbon atom
- one oxygen atom
- two nitrogen atoms
- four hydrogen atoms
You know for a fact that the ammonium ion,
- one nitrogen atom
- four hydrogen atoms
Well, if urea is the only product of the decomposition reaction, then all of the atoms that are a part of the urea molecule must have been a part of the ammonium cyanate - think the Law of mass conservation.
More specifically, since, four hydrogen atoms and one nitrogen atom are accounted for by the ammonium ion, you get that the cyanate ion must have contained
- one carbon atom
- one oxygen atom
- only one nitrogen atom
Therefore, you can say for sure that balanced chemical equation for this reaction was
#"NH"_4^(+) "CNO"^(-) stackrel(color(red)("heat"))(->) "CO"("NH"_2)_2#
or, if you want to write the complete process, you have
#"NH"_4^(+) "CNO"^(-) stackrel(color(red)("heat"))(->) "NH"_3 + "HCN" -> "CO"("NH"_2)_2#
Check out this very cool gif I found of the reaction