What is the common oxidation state of oxygen atom?
1 Answer
Oxygen is in the 14th column in the periodic table.
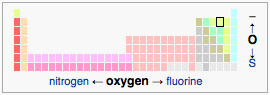
Given the elements in the p-block (such as
In order to become more stable, oxygen wants to lose or gain electrons so that it achieves an "octet," meaning a full set of 8 electrons in its highest-energy orbitals. To do so, neutral oxygen (
Each electron adds one (

