What are possible structures for an organic molecule whose mass spectrum gives m"/"z=128, 85, 71, 57, 43?
2 Answers
This could be anything (well anything with a mass of 128 amu). You need to do more experiments.
Explanation:
What is the melting point of the hydrocarbon? What are the melting points of its derivatives? You will not identify an unknown organic compound with only a mass spectrum.
I'm guessing that one possibility is 2-methyloctane.
Explanation:
You know that the compound is a hydrocarbon.
Let's assume that it's an alkane. Then its molecular formula is
Its molecular mass is
So the molecular formula is
The base peak at
The other fragments must be less stable (1°?).
The peaks at
A possible structure is 2-methyloctane
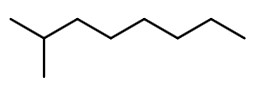 Parent
Parent
If the molecule cleaves between
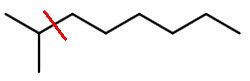 Scheme 1
Scheme 1
we get an isopropyl fragment (
If the molecule cleaves between
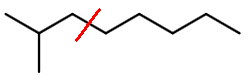 Scheme 2
Scheme 2
we get a 1° isobutyl fragment (
The isobutyl cation should be more stable because of the inductive effect of the isopropyl group.


